Abstract
Sickle Cell Disease (SCD) is an inherited hemoglobin disorder which causes a mutation in the β subunit of hemoglobin (HbA), thus producing hemoglobin S (HbS) (Williams and Thein, 2018). When HbS is deoxygenated, red blood cells (RBC) can polymerize, which promotes increased rigidity and microvascular occlusions. The presence of mutated HbS increases the likelihood of recurrent venous thromboembolism (VTE). There is an unmet need for real-time, non-invasive methods of assessing microvascular hemodynamics and endothelial dysfunction. Near-Infrared Spectroscopy (NIRS), is a non-invasive, optical technique which measures tissue absorption and scattering between 650-1100 nm in subcutaneous tissue. NIRS has the capability to quantitatively measure the concentration of oxy- and deoxy- hemoglobin as well as blood flow. To determine the sensitivity of NIRS to changes in hemodynamics in SCD patients, we evaluated a cohort of SCD patients undergoing treatment with Isoquercetin (IQ), a flavonoid antithrombotic agent, and correlated the findings with D-dimer, a marker for blood clot degradation.
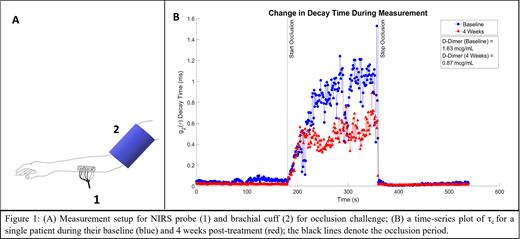
We recruited a sub-cohort of 17 of 46 patients participating in a randomized, double-blind, placebo-controlled trial of IQ and performed an optical hemodynamic assessment at baseline and 4 weeks post-treatment. We used the commercial, multi-modal NIRS device (MetaOx, ISS Inc) which combines frequency-domain NIRS (FD-NIRS) and diffuse correlation spectroscopy (DCS) to acquire optical markers of tissue composition and blood flow, respectively. NIRS testing included resting baseline measurements and a brachial cuff occlusion. As seen in Figure 1A, a blood pressure cuff was wrapped around the bicep and the NIRS probe was placed on the ipsilateral forearm muscle. The challenge consisted of a 3-minute baseline, 3-minute cuff occlusion at 200 mmHg, and a 3-minute recovery.
The decay constant τc of the intensity autocorrelation function g2(τ), which is an indicator of the bulk displacement of scattering particles (i.e. RBCs) in the forearm, was fitted from the DCS data. We compared the mean τc during the last 30s of the occlusion to D-dimer levels for all 17 patients at baseline and at 4 weeks follow up. Following a data quality review, 8 patients had NIRS data for baseline and follow up visits. D-dimer levels were 1.875 mcg/mL ± 0.869 mcg/mL at baseline and 1.449 mcg/mL ± 0.874 mcg/mL at 4 weeks follow up. Among the 8 patients with good quality data, 5/8 showed an average decrease in τc by 0.424 ms at 4 weeks follow up and an average decrease in D-dimer levels by 0.768 mcg/mL. The patient in Figure 1B showed 42.0% decrease in τc and 46.6% decrease in D-dimer levels from baseline to 4 weeks follow up. 2/8 patients showed an increase in τc by an average of 0.139 ms and an average increase in D-dimer levels by 0.320 mcg/mL. 1/8 patient showed a decrease in the τc with no change in D-dimer levels. We found that 7/8 patients with baseline and follow up visits had matching trends between D-dimer levels and τc. As demonstrated, a larger τc signified less movement of scattering particles, such as RBCs, due to increased blood coagulation. The remaining 9/17 patients had data from one visit of acceptable quality and were excluded from this analysis.
The results of this initial analysis demonstrate autocorrelation decay time could be a promising metric that is reflective of blood coagulation and rheology. While this analysis focused on the changes observed in optical blood flow markers, further work will be performed to both expand upon this analysis and to integrate the compositional data acquired from the FD-NIRS portion of the measurements. NIRS can potentially complement traditional blood biomarkers when assessing anti-thrombotic effects of SCD treatments, such as Isoquercetin.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal